|
Radioactive equilibrium Any member of a decay chain is produced from the decay of the preceding member. Therefore, as many new nuclei are formed in a unit of time, as the number of decays from the preceding member. Denote a decay chain by 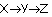 , and the corresponding activities (number of decays in unit of time) AX, AY, AZ . The member Y is produced by the decay of X, therefore the production rate of the Y equals the decay rate of X, i.e. Ax . , and the corresponding activities (number of decays in unit of time) AX, AY, AZ . The member Y is produced by the decay of X, therefore the production rate of the Y equals the decay rate of X, i.e. Ax . The decay rate of Y is determined by its own activity, i. e. AY. Equilibrium is reached, when the production rate and the decay rate of Y become the same, ie. if AX = AY. The reasoning is valid for any internal member of the decay chain. Therefore, the activities of the members of a decay chain are equal in radioactive equilibrium
… AX = AY = AZ = … The activity is 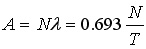
(here 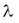 is the decay constant, N is the number of the nuclei and T is the half-life). Therefore for a radioactive equilibrium we get is the decay constant, N is the number of the nuclei and T is the half-life). Therefore for a radioactive equilibrium we get 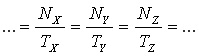
It follows that in a bulk of material (e.g. ore) where radioactive equilibrium is maintained, the quantity (concentration) of the different members are proportional to their half-lives (large concentrations for large half-lives, and small concentrations for short half-lives).
|








