|
Glossary Activity The activity of a sample is defined as the number of decays in a unit of time. (The time-unit should be much shorter than the half-life).
The unit of the activity in the SI system is: becquerel
Denoted by: Bq
1 Bq = 1 decay/s. The activity of a sample is proportional to the number of the nuclei N in the sample and to the decay constant 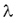 . . 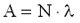
Since the number (N) of the radioactive nuclei in a sample follows the exponential decay law, the activity of the sample is also decreasing at the same rate. Alpha-decay In the alpha-decay a helium-ion (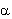 -particle, 4He) is emitted, therefore the atomic number of the decaying nucleus decreases by two, and the mass number decreases by four. -particle, 4He) is emitted, therefore the atomic number of the decaying nucleus decreases by two, and the mass number decreases by four. 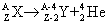
Decreasing the mass number of light elements is not an energetically favorable process, therefore, alpha-emitters can be found only among the heavy elements. Beta-decay Beta particles are electrons emitted from the nucleus; the term "beta particle" is a historical term used in the early description of radioactivity. There are three types of beta-decay:
- Negative beta decay
- Positive beta decay
- Electron capture
Only the negative beta decay occurs in natural radioactive decay chains, therefore only this type will be discussed below. Negative beta-decay During the negative beta decay a neutron of the nucleus is transformed into a proton, while an electron (e-) is emitted. (During the decay also a small neutral elementary paricle – antineutrino - is emitted, but this particle can be neglected from a practical point of view, because this particle interacts very weakly with the matter). The negative beta-decay is desribed by the following equation: 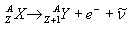
In the negative beta-decay the atomic number of the nucleus increases by one and the mass number remains constant. Decay constant The decay of radioactive materials is a random process. The probability that a particular nucleus will decay in a unit of time (e.g. 1 s) is called decay constant. The decay constant is usually denoted by : 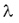 The unit of the decay constant is (in SI units): 1/s. Small value of the decay constant results in slow decay, large value of the decay constant results in fast decay. If the decay constant is known, the activity of the material can be determined by the expression: 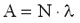
Here A represents the activity of the material (number of decays in a unit of time), N is the number of the radioactive nuclei in the sample. The decay constant is also related to the half-life: 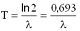
T is the half-life of the sample. Gamma-decay In the gamma-decay the nucleus emits electromagnetic rays (called 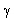 -photons), therefore this process is similar to the light emission of atoms. -photons), therefore this process is similar to the light emission of atoms. However, there are differences between the visible light emission and gamma ray emission! First, the gamma photons are emitted by the nucleus, whereas the electron shell of the atom emits the visible light. Second, the energy of the gamma-photons is much higher than that of the visible photons. Therefore, the wavelength of the gamma rays is much shorter than that of the visible light. Half-life The amount of time necessary for the half of a parent isotope to turn into its daughter isotope is called half-life. The expected number of nuclei N(t) in function of time (t) can be calculated by the following mathematical expression. 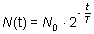
Here t denotes the time, N0 is the number of nuclei at the beginning (t=0) and T is the half-life. The function is shown below in the graph. 
The half-life and the decay constant are inversely proportional to each other: the smaller is the decay constant, the more time is required for the decay of the half of the sample. 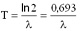
Here T is the half-life, 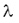 is the decay constant. is the decay constant. The half-life is characteristic of the isotope. The half-life of the different radioactive materials can range from several billion years to billionth of a seconds. The probability that a particular nucleus decays during T1/2 time (T1/2 is the half-life) is exactly 1/2. The decay probability during the next T time period is again 1/2, and it is independent since how long the particular nucleus is “waiting” already for the decay. Isobar Isobars have the same mass number, but their atomic numbers are different. Isobar atoms can be transformed into each other with beta-decays. Izotope Isotopes have the same atomic number, but their mass numbers are different, since they have different numbers of neutrons. The chemical and biological behaviour of the isotopes are completely the same. However, they are different particles and they have different nuclear physical properties. This was discovered by George de Hevesy (01. 08. 1885. Budapest – 05. 07. 1966. – Freiburg – Chemical Nobel prize in 1944), and it has been used for the method of radioactive traceing. 
Mass number The nucleus is composed from protons and neutrons. Their common name is “nucleon”.
The number of protons (usually denoted by Z) is the atomic number of the element. (This corresponds to the place of the atom in the periodical table of elements). A = Z + N The total number of the protons and neutrons is called mass number, and it is denoted by A.
Usually the mass number is written as left-side superscript to the chemical symbol of the atom. For example  denotes an uran (U) nucleus with 92 protons inside (left-side subscript), and containing 238 particles in total (left-side superscript). denotes an uran (U) nucleus with 92 protons inside (left-side subscript), and containing 238 particles in total (left-side superscript). Neutron It means “neutral” in Latin. The neutron is an electrically neutral elementary particle. Usually it is denoted by n. Together with the proton the neutron can be found in every nucleus (exception: the light isotop of the hydrogen). The common name of the neutron and the proton is “nucleon”. Mass: 1,674920* 10-27 kg = (1,00866497±0,0000004) u. Proton It means “first” in Greek. The proton is a stable elementary particle, the nucleus of the light hydrogen atom. Mass: 1,672614* 10-27 kg = (1,007264433 ± 0,0000008) u, Töltése: 1,6021917* 10-19 C (egyszeres pozitív elemi töltés). Charge: 1,6021917* 10-19 C (positive elementary charge).
|








