Wave Functions of an Electron on a String and EM
transitions
The electron on a
string is one of the simplest models where the basics of quantum mechanics can
be demonstrated. Unfortunately in high school one can use only real functions,
which immediately causes some problems if one tries to answer some of the questions
of a smart and interested student. Some of these “difficult” questions are the
following:
• The wave function is usually represented as
vibrations of a string. The square of the wave function shows “where” the
electron is located (finding probability distribution). However, during the
vibration there are moments when the displacement of the string is zero
everywhere. Where is the electron in such a moment? Has it disappeared?
• The oscillation frequency of the wave
function is known: E=h∙f. If the electron is oscillating like this, it should radiate energy
like an antenna. Why electrons do not radiate in the eigenstates of the atoms,
if their wave functions are oscillating? (The usual answer is that the electrons
are “standing” still, only the wave function is oscillating.)
• How can one imagine that the wave functions
are oscillating, but the electrons are standing?
• How can an electron emit or absorb electromagnetic
radiation? Does it behave like an antenna? How can it do that, if it stands
still in all atomic states?
This simulation gives
some hints to understand these phenomena and to answer these questions.
Short theoretical summary
According to the
quantum mechanics the wave function of an electron on a string is the following: 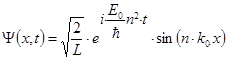 .
.
Itt ![]() ,
, ![]() ,
,
L is the length of the string, m is the electron mass, and n = 1, 2, 3, 4… are the quantum numbers of the state.
Obviously, one cannot
write down complex functions in high
school! Instead, we can say that the state of a particle will be described
by two (real) functions. Let us
denote them Reѱ and Imѱ.
These can be imagined as components of a vector. Then the (Reѱ)2+(Imѱ)2
expression shows where the particle can be found. This might seem familiar also
to students in high school, since they learned already how to calculate the
length-square of a vector: by adding the
squares of its components.
It is easy to see
from the above complex expression that
![]() , and
, and ![]() .
.
This simulation helps
to show the following:
1) To illustrate the two components of the wave function (Reѱ and Imѱ
), including their time behavior. Their frequency can be
calculated as ![]() , where
, where ![]() . This „illustration” can be done by drawing
the real (Reѱ) and
the imaginary part (Imѱ) separately
(2D drawing),
but it is also possible to draw them in a 3D coordinate system
(x, Reѱ, Imѱ) .
. This „illustration” can be done by drawing
the real (Reѱ) and
the imaginary part (Imѱ) separately
(2D drawing),
but it is also possible to draw them in a 3D coordinate system
(x, Reѱ, Imѱ) .
2) It is also possible to draw a
wave function composed as a superposition
of two arbitrary selected wave functions![]() .
.
The drawing can be performed as well
in 2D as in 3D.
3) The simulation shows also the time and space
behavior of the finding
probability ![]() of the mixed state, as well as the position of its „center of mass”.
of the mixed state, as well as the position of its „center of mass”.
(The finding probability of one single state can
be seen, if one “mixes” the same state.)
It is interesting to observe
a) The wave functions of the
eigenstates are oscillating, but the distributions of their finding
probabilities as well as their centers of mass are constant. Therefore the
electrons in these states are not “moving”, therefore they do not radiate.
b) The center of mass of certain “mixed”
states is oscillating.
According to the electrodynamics an oscillating electrical charge (electron)
radiates like an antenna.
c) It can be observed that the
oscillation frequency is ![]() , i.e. the difference of the two original frequencies. (Please
note that the amplitude and the frequency of the oscillation will be determined
by the simulation, analyzing the resulting motion of the center of mass, and
not using the above formula! Therefore one should wait a few oscillations until
the correct amplitude and frequency will be shown.) Using the
, i.e. the difference of the two original frequencies. (Please
note that the amplitude and the frequency of the oscillation will be determined
by the simulation, analyzing the resulting motion of the center of mass, and
not using the above formula! Therefore one should wait a few oscillations until
the correct amplitude and frequency will be shown.) Using the ![]() formula we get
formula we get ![]() . This is Bohr’s result for the frequency of the emitted
photon.
. This is Bohr’s result for the frequency of the emitted
photon.
d) It can be seen, that only those superpositions lead to an oscillation of the center of
mass, where the symmetry of the two components are different. There is no
oscillation for
n = 1 and n = 3 mixture, or for the n = 2 and n = 4 mixture. This is the reason (in the case of the 3D atoms)
that there is no photon emission at a 2s → 1s decay (since both quantum
states are spherically symmetric, therefore the center of mass does not change
at their mixture).