The composition of the nuclei Nuclei consist of protons and neutrons.
Nuclei are characterised by the number of protons in them (atomic number, denoted by Z) and the sum of the protons and neutrons (mass number, denoted by A).
Usually the mass number is written as left-side superscript to the chemical symbol of the atom, whereas the atomic number is written as left-side subscript.
For example, 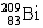 denotes a bismuth (Bi) nucleus, whith 83 protons inside (left-side subscript), and which containing 209 particles in total (left-side superscript). From these values the number of neutrons can also be easily determined: 209 – 83 = 126 denotes a bismuth (Bi) nucleus, whith 83 protons inside (left-side subscript), and which containing 209 particles in total (left-side superscript). From these values the number of neutrons can also be easily determined: 209 – 83 = 126 Naturally, the chemical symbol already determines the number of protons in the nucleus, therefore the atomic number can be omitted in certain cases. Two types of this shorter notation are commonly used: 209Bi, or Bi-209. If needed, the atomic number of the element can be determined from the periodic table using the chemical symbol of the element. | 







