During the alpha-decay a helium-ion (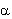 -particle, 4He) is emitted. -particle, 4He) is emitted.
Rutherford found in 1909 that the alpha-particles emitted in the alpha-decay process are highly energetic helium ions. Therefore the atomic number of the decaying nucleus decreases by two, and the mass number decreases by four in an alpha-decay. The range of the alpha-radiation is very short. The particles reach only a few centimetres in the air, their range is about tenth of a millimeter in a solid material. | 







